Menu
Platform Technologies
Brain Disease
Cancer Therapy
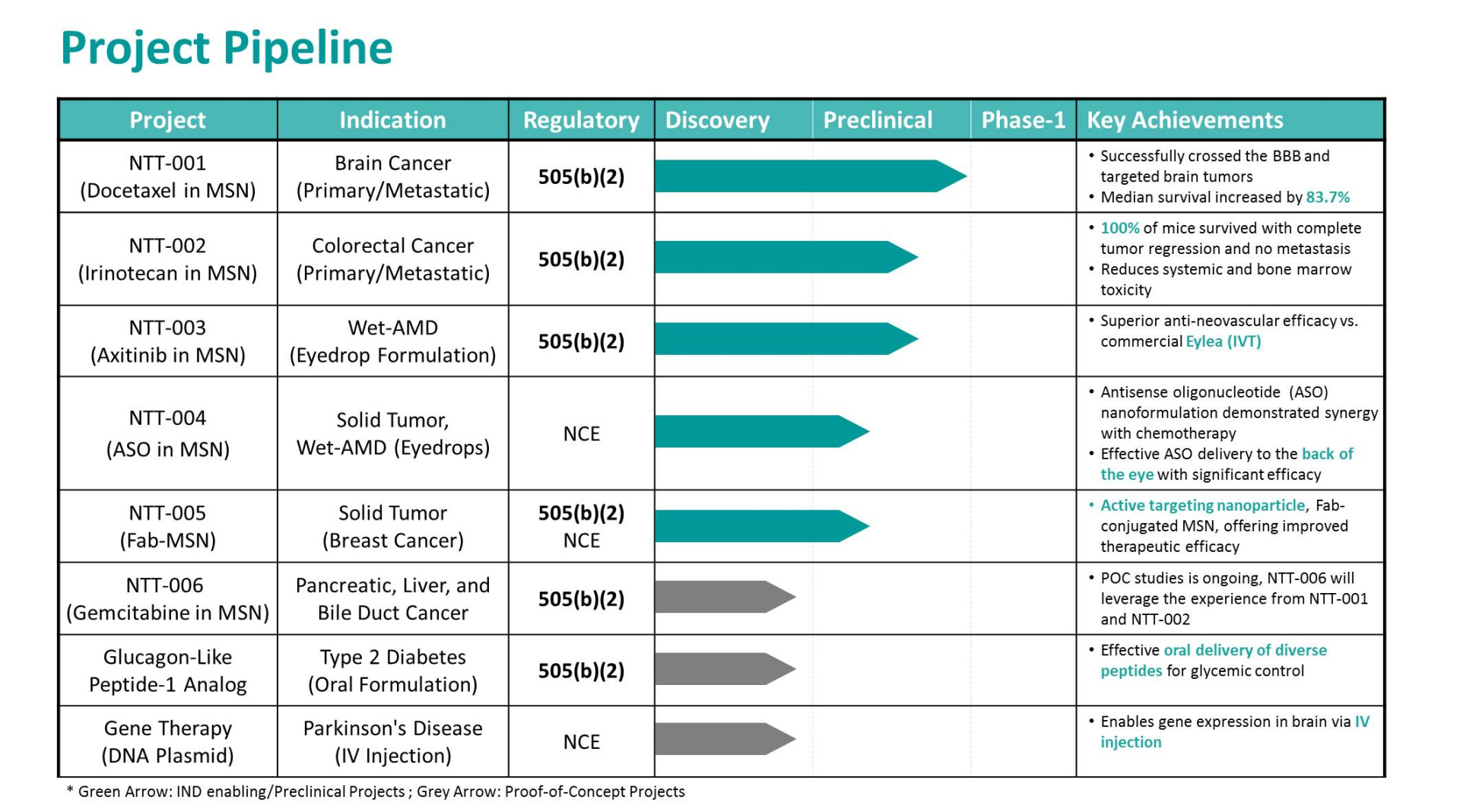
NTT-001
Summary
• NTT-001 is a nanoformulation of docetaxel-loaded mesoporous silica nanoparticles (MSNs) developed for the treatment of primary and metastatic brain cancers. This advanced nanomedicine is designed to cross the blood-brain barrier (BBB) and selectively target brain tumors, addressing critical limitations of current brain cancer therapies.
• A Phase 1/2 clinical trial is anticipated to begin in early next year.
BBB Penetration and Tumor Targeting
NTT-001 selectively accumulates in brain tumors by crossing the compromised blood-brain barrier, while showing minimal penetration into healthy brain tissue.

Efficacy of Tumor Growth Inhibition
In an orthotopic temozolomide-resistant brain tumor model, NTT-001 treatment significantly reduced tumor volume and extended median survival by 83.7% compared to both the control and docetaxel (DTX) groups

Pharmacokinetics and Toxicology
Pharmacokinetics:
• NTT-001 demonstrated higher systemic exposure, as indicated by increased Cmax and AUC0-t, compared to the DTX group.
Toxicology:
• NTT-001 showed milder histopathological changes compared to the severe toxicity observed in the DTX group.
• No significant toxicity was observed in animals treated with the MSN alone.
Further details can be found in our publications:
ACS Appl. Mater. Interfaces 2024, 16, 21722
ACS Nano 2024, 18, 12716